[ Instrument Network Instrument Development ] Liu Guosheng, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, developed the acene carbon-hydrogen bond (including high-site, high enantioselective) cyanidation of complex olefins. The reaction, in cooperation with the Hong Kong University of Science and Technology Lin Zhenyang research group, through the combination of experimental and theoretical calculations, revealed a new mechanism for the selective oxidation of nitrogen by metal-regulated nitrogen radicals. The work was published online October 24 in the journal Nature. Liu Guosheng's doctoral student Li Jiayuan is the first author of the thesis, and Shanghai Organic Institute is the first unit.
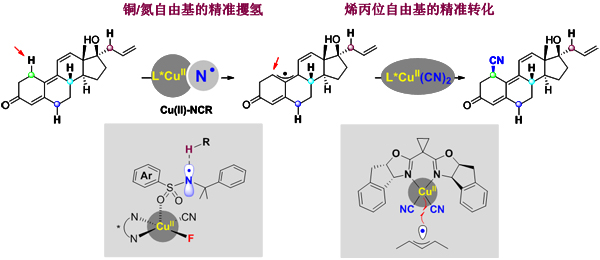
Hydrocarbon bond activation is the holy grail of organic chemistry, and the precise conversion of organic molecules based on carbon-hydrogen bond activation is the jewel in the Holy Grail, which has been the focus of synthetic chemists. The hydrogen atom transfer (HAT) strategy of free radicals is an effective way to achieve carbon-carbon bond functionalization and has been extensively studied. However, in order to achieve accurate conversion of organic molecules, there are two very important scientific issues to be solved: (1) How to achieve the free radical selective hydrogenation of carbon-hydrogen bonds in organic molecules? Previous studies have been carried out based on carbon-hydrogen bonds with significant differences in organic molecules, while structurally similar hydrocarbon bonds are difficult to achieve selective hydrogenation; (2) How to control the asymmetric transformation of carbon radicals after hydrogen-hydrogenation? Due to the high activity of free radicals, the control of asymmetric transformation is very difficult; it is these two scientific problems, such as the existence of two mountains, leading to the precise conversion of organic compounds' carbon-hydrogen bonds. Only by breaking through the above two scientific problems can we achieve precise functionalization of carbon-hydrogen bonds.
In order to explore the precise transformation of carbon-hydrogen bonds, Liu Guosheng's research group conducted research. In 2016, they collaborated with Stahl, a professor at the University of Wisconsin in the United States, for the first time to propose a new concept of copper-catalyzed free radical bonding, achieving asymmetric cyanation of the benzylic CH bond, revealing the chiral bivalent copper cyanide. Species can effectively capture benzylic free radicals, and obtain chiral nitriles with very efficient and high enantiomers, thus achieving direct conversion from simple petrochemicals to drug precursors (Science 2016, 353, 1014) This not only answers the second scientific question mentioned above, but also lays a solid foundation for the study of the first scientific issue.
The ally-position carbon-hydrogen bond is similar to the bond energy of the benzylic hydrocarbon bond (BDE: ~83 and ~85 kcal/mol), both of which belong to the active carbon-hydrogen bond category, thus achieving an asymmetric cyanide in the ally-position carbon-hydrogen bond. The reaction is expected. However, since olefin molecules often contain a plurality of olefinic hydrogen atoms, a plurality of olefins are often present in biologically active molecules (natural products, drugs, etc.); therefore, the presence of a plurality of allylic carbon-hydrogen bonds leads to free radicals. The problem of hydrogen selectivity; the simultaneous formation of allyl radicals also has problems of region, steric and enantioselectivity in subsequent reactions, making the reaction extremely complicated. In order to explore the problem of selective hydrogen hydration of allylic carbon-hydrogen bonds, Liu Guosheng's research team and Lin Zhenyang's research team first discovered that metal copper species can coordinate with nitrogen radicals containing sulfonamides (Cu-bound N-centered radicals). , Cu-NCR), thereby adjusting the hydrogen-hydrogen ability and selectivity of nitrogen radicals, achieving a site-specific HAT of high-site selective ally-hydrocarbon bonds; and from a theoretical calculation point of view A new mechanism for the selective oxidation of nitrogen by metal-regulated nitrogen radicals is described. This discovery provides a new way of thinking for the later study of the selective conversion of hydrocarbon bonds. What is even more gratifying is that the allylic radicals obtained by free radical hydrogen can also be captured by the chiral copper cyanide species in the system, and the single chiral cyanidation is also obtained with high region and high enantioselectivity. The product thus achieves precise conversion of complex olefin molecules. It is very important that the reaction system not only has a very broad substrate universality and functional group compatibility, but also is suitable for the precise modification of complex drug molecules in the later stage, and provides a new way for the development of new drugs and the modification of drug molecules. This research is another breakthrough of Liu Guosheng's research group on the basis of their preliminary quaternary carbon-hydrogen bond asymmetric cyanidation research.
The work was funded by the Ministry of Science and Technology, the National Natural Science Foundation of China, the Chinese Academy of Sciences, the Shanghai Municipal Science and Technology Commission, the Shanghai Organic Institute and the State Key Laboratory of Metals and Organics.
Forged Parts,Aluminum Forged Parts,Forged Components,Cold Forged Components
Suzhou SNK Machinery Equipment Co.,LTD , https://www.snkforgedroll.com
